3D-printing
Currently, large pharmaceutical companies mass-produce the same version of a drug and deliver these to various patients. This “one-size-fits-all” approach is restrictive because not all individuals react in the same way to diseases and to a pharmacological treatment (dependent on factors such as age, sex, bodyweight). Here, personalized medicine can make a ground-breaking change in the treatment of patients. When it comes to manufacturing a variety of specific dosages on a small scale, industrial machines that are dedicated to mass production may not be the best option. With the emergence of 3D printing, the idea of printable medicines arose, enabling the production of a wide range of dosages, forms, designs, and geometries. Nevertheless, it is quite a challenge to apply this highly promising technology in the world of GMP environments. With years of experience across all facets of GMP, Advipro has all the required expertise to facilitate the implementation of 3D printing technology in the pharmaceutical industry and beyond.
Opportunities
Although 3D printing of pharmaceutical drugs is still in its early stages, it provides great opportunities towards personalized medicine. We discuss several of these opportunities below.
Dose personalization
For young children and older generations, dosing requirements can be markedly different compared to adults due to differences in physical characteristics (e.g. body weight, surface area, and age) and pharmacokinetics (e.g. metabolic capacity and organ function) [1]. Furthermore, certain medicines with complex dosage regimes require exact dosing to maintain treatment efficacy and patient safety. As such it has become common practice for patients to split tablets or for medical staff to prepare formulations to achieve the right dose. Such practices can have the risk of inaccurate doses and dose variation for the patient. In these cases, 3D printing could be used to create a “printlet” containing an exact dosage of a drug. In turn, this could simplify administration and reduce the risk of dose variation and medication errors. An example of dose personalization is the antiepileptic drug Spritam manufactured by Aprecia, which is the first and only drug regulated by the FDA that uses 3D printing. The printing process takes place using a patented technique which allows it to easily adjust doses.
Multi-drug combinations
As the population ages and rates of chronic disease rise, an increasing number of people are taking multiple pills for several conditions, often at different times throughout the day. Taking the right pill at the right time can be a hassle and potentially dangerous if a mistake is made. This is especially true for people with dementia. It would be more convenient and safer if people could take just one pill a day that delivers all the right medication at the right time in the right dose. These “Polypills” could have many layers that are released at different times in the human body. It is not difficult to mass-produce these polypills. But there is a drawback, a polypill with a specific combination of drugs does not necessarily help all patients. Some people might not need one of the drugs or they might need them in different doses to those in a mass-produced polypill. Making personalized polypills through the usual drug manufacturing techniques would be very difficult. A much cheaper way of making these polypills is 3D printing, in which thin layers of (different) materials are built up according to the design to make a final product.
Tailored release profiles
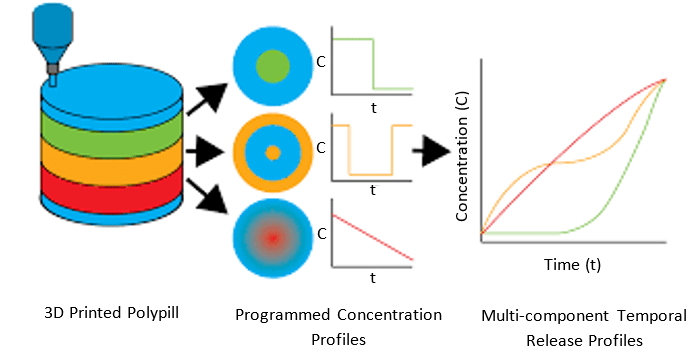
In line with the multi-drug combinations, by selecting the right excipients (polymers) and printing parameters, printlets could also be developed to have defined drug release profiles. Because of this, just one pill a day can give the right dose at the right time to the patient. An example of such a release profile can be found in Figure 1.
Figure 1: example release profile of a polypill. Adapted from [2]
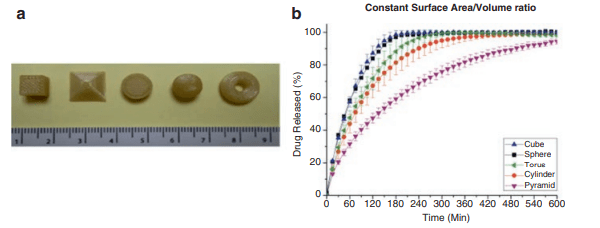
It has also been shown in the past that release profiles could also be influenced by changing the geometry of the tablet [3]. According to a study of the Department of Pharmaceutics at the University College of London, modification of drug release is possible by changing the surface area to volume ratios of different shaped printlets [4]. These complex shapes can be easily obtained by 3D printing.
Figure 2: release profiles of different geometries: Adopted from [3]
On demand printing
3D printing has the advantage of on-demand and immediate production of medicines. It could be integrated across various healthcare services, ranging from pharmacies to hospital departments. This could change access to medicine and transport costs drastically. This is particularly useful for products with limited shelf life. Also, areas with poor medicines access could benefit from 3D printing. Think of cases such as military operations, third-world countries, disaster areas, and even space missions [1]. The ability of producing the required medicine at the required time can be a huge benefit. However patient safety is paramount, which is why the production of pharmaceuticals has to be fully GMP compliant. This is where Advipro’s employees could use their experience in, and knowledge of compliance, to ensure the production of safe pharmaceutical products.
Drug development
Clinical studies are a permanent part of drug development, consisting of different phases. During these studies, a wide dose range is often administered to gain initial information around safety, tolerability, and toxicity. These types of studies take up the most time and cost in a drug development process. Also, less than 10% of drugs in phase I trials were approved by the FDA. Changes in the formulation are still very expensive and take up a lot of time in clinical trials, this is because current manufacturing processes are often very inflexible and complex. 3D printing could be a game-changer for this. Thanks to this technique, changes can be responded to very quickly. This can drastically reduce the cost and duration of clinical trials. According to some studies [1], the costs of drug development can be reduced by up to 70%. The biggest savings are in the reduction of API (thanks to smaller badges, up to 50% can be saved on API during phase 1 studies) and reduction of working hours (due to the flexibility of 3D printing in formulation, trading can be done up to 60% faster). Due to the compact size of 3D printers it could be possible, with the right knowledge of GMP production, to integrate these systems into clinical trial and laboratory settings. This again is a process where Advipro can get involved to facilitate the implementation of 3D printing compliant to GMP regulations.
Validation of 3D drug printing
3D printing of drug products should be, just like other production techniques, performed according to GMP guidelines. To validate 3D printing, the process of 3D printing should be understood. The process is not only the act of 3D printing itself, but it also consists of software. Thus, the process can be subdivided into a software workflow and a printing workflow.
Advipro is specialized in validating state-of-the-art equipment and cleanrooms in highly regulated environments, such as the pharmaceutical industry. Which is why Advipro can boost this technological advancement by providing services to install and properly validate 3D printers and the cleanrooms they are located in.
Computer system validation (CSV) is also a large part of the validation plan (VP) of 3D printers since there is a lot of software involved in this process. CSV occurs according to GAMP 5 regulations. Solutions containing this CSV can be provided by Advipro as well. Numerous projects in large pharmaceutical companies have been completed with the help and good work of Advipro employees.
Aside from equipment and computer system validation, other aspects of the manufacturing process, such as process, cleaning, and sterilization validation are within the scope of Advipro’s expertise.
Conclusion
To conclude, 3D printing can be a ground-breaking step towards personalized healthcare and medicinal drugs. This technology has been proven to be effective, yet it still has a long way to go to be used around the world. The adoption of 3D printing of medicinal drugs is a challenge for pharmaceutical companies. To help reach this 3D printing’s full potential, Advipro can provide solutions in adopting this technology and helping the industry in taking a step towards more suitable and personalized healthcare.
References
- Basit, A. and Gaisford, S., 2018. 3D Printing of Pharmaceuticals. 1st ed. [ebook] Springer, Cham. Available at: https://doi.org/10.1007/978-3-319-90755-0.
- Haring, A. P., Tong, Y., Halper, J., Johnson, B. N., Adv. Healthcare Mater. 2018, 7, 1800213. https://doi.org/10.1002/adhm.201800213.
- Abaci, Alperen & Gedeon, Christina & Kuna, Anna & Guvendiren, Murat. (2021). Additive Manufacturing of Oral Tablets: Technologies, Materials and Printed Tablets. Pharmaceutics. 13. 156. 10.3390/pharmaceutics13020156.
- Iria Seoane-Viaño, Patricija Januskaite, Carmen Alvarez-Lorenzo, Abdul W. Basit, Alvaro Goyanes. (2021). Semi-solid extrusion 3D printing in drug delivery and biomedicine: Personalised solutions for healthcare challenges, Journal of Controlled Release, Vol. 332. https://doi.org/10.1016/j.jconrel.2021.02.027.