

The three pillars of process validation: An insightful exploration

- Aline Acke
To meet patients' needs, scientific laboratories conduct intensive research to develop new and improved drugs. But what happens after a new product is discovered? Can it just hit the market, and is it reliable? The drama of the Softenon babies may come to mind when we think of newly developed drugs.
Fortunately, since the 1960s, there have been much stricter requirements and pharmaceutical companies strive to bring quality medication to the market. Important in this is the complete mapping, evaluation and continuous control of the production process, also known as process validation, an essential aspect within the pharmaceutical sector.
In this informative blog, we dive deeper into the world of process validation, a crucial component within the pharmaceutical industry.
Introduction
The validation of manufacturing processes is very important to ensure both the quality and effectiveness of the final products and to prove that the process is in full control.
Since process validation covers the entire process and operation, an interdisciplinary approach is essential. By getting different teams such as engineering, microbiology, statistics, manufacturing, quality assurance (QA), and so on working together, information about the life cycle of a product or process can be studied and interpreted from different perspectives.
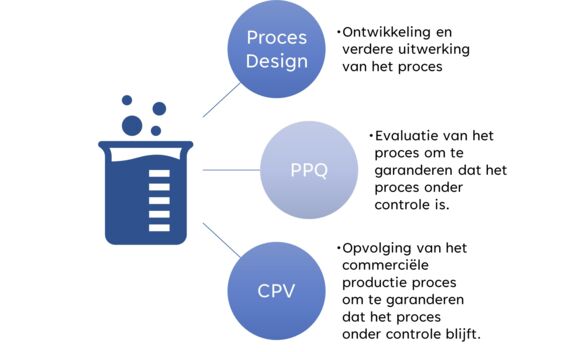
Process validation and all its facets involved can therefore be divided into three main pillars: Process Design, Process Performance Qualification and Continued process verification. Each of these pillars will also consist of several steps, with a correct but above all safe product as final output.
Process validation comprises three main pillars:
Pillar 1: Process design - building knowledge
We start our road to a commercial product by building process knowledge through scientific experiments, usually carried out on a smaller scale in laboratories. Together with information previously gathered during product development, these experiments serve to achieve large-scale production and understand the parameters that influence both product quality and process performance.
After gathering process knowledge, a strategy is developed to control the process. Controls include analysis of (intermediate) product samples and monitoring of used equipment with alarm systems during critical process points.
Risk assessments are carried out to identify steps with more risks, which require closer monitoring. As a result, all process parameters are classified from highly critical to less critical, with evaluation on product impact depending on the deviation of a given parameter from its established value.
Pillar 2: Process Performance Qualification (PPQ) - Striving for perfection
After gathering sufficient process knowledge and assessing the critical aspects, we move on to process qualification. From this point, it is essential to comply with Good Manufacturing Practices (GMP), which was previously not mandatory according to government agencies such as the FDA and EMA.
The first step in this phase is the qualification of the equipment used, such as (bio)reactors, freezers and rooms. After successful completion of this facility qualification, effective process qualification (PPQ) can start.
During PPQ, all parameters previously identified as critical are closely monitored. Compared to routine production, more samples are taken, and additional tests performed to confirm with high certainty that the process is under control. The PPQ protocol should include:
- A strategy with all criteria and process performance parameters*, including a statistical description of data analysis to trend variations, and procedures for dealing with deviations and non-conform data.
- Specifications for the data to be collected.
- Tests to be performed, with criteria for considering a test 'passed'.
- A sampling plan that includes all samples to be taken during each process step.
- Validation status of analytical methods used on (intermediate) products.
- Review and approval of all departments involved and quality (QA).
Using this protocol, PPQ runs are then performed and recorded in a report with all data and information obtained. Often, PPQ is considered successful when three consecutive runs are successfully completed.
*Process performance parameter is a metric used to measure the performance of a process. Ideally, you want to have steering information that you can use to steer the process in the right direction.
Pillar 3: Continued process verification (CPV) - The process remains under the microscope.
With a successful completion of PPQ, the product can finally be commercially produced and marketed. However, process monitoring does not stop here. During each production run, samples are still taken and analyzed to quickly detect and correct possible variations or atypical trends in the process.
Through statistical trending, both process quality and performance are closely monitored against the previously obtained process parameters that affect process control and therefore the final product.
The figure provides a summary of the three pillars:
Conclusion: Guarantee of quality and safety:
The three pillars of process validation ensure that pharmaceutical companies can consistently deliver products of the highest quality. This not only guarantees that medicines are effective, but above all safe for use.
Did you know that...
Our MoVE team is ready every day to contribute to the second and third pillar? Our colleagues have expertise in sampling and testing, but also in qualification to ensure that your equipment or space (clean room, warehouse, ...) complies with the legal requirements with an eye on quality and safety.
Could you use some support? Contact us at move@advipro.com, Nathan, associate business manager of MoVE, will gladly look at the possibilities with you.
Do you want to contribute to safe medication?
Were you triggered by reading this blog and are you eager to contribute to the pharmaceutical world? Then take a look at our vacancies via the button below, and who knows, maybe you will soon be working on maintaining safe medication in our society!

