

Regulatory Affairs: Safe, effective and high quality medicinal products on the market
The development and marketing of medicinal products is a highly regulated process involving continuous interaction between the pharmaceutical company (the "sponsor") and the competent health authorities. On the sponsor’s side, regulatory affairs is responsible for ensuring compliance with laws and guidelines, relating to the safety, efficacy and quality of medicinal products.
In this blog, we will have a look at the regulatory processes in three key phases of the drug lifecycle, focusing on the EU region. First, as part of the drug development process, the sponsor must submit an application to the competent health authority and obtain approval before conducting a clinical trial. Then, once the clinical trials have demonstrated that the new drug is safe, effective and of high quality for the intended patient population, the marketing authorization application (MAA) dossier can be submitted to the competent health authority. If the decision is positive, the company may release the drug on the market and make it available to patients. Finally, during the commercial phase, changes to the product may occur. If these have a direct impact on the content of the marketing authorisation (MA) dossier, a variation procedure must be followed.
1. Regulatory aspects in Clinical Development
The purpose of clinical development is to investigate the safety and efficacy of a drug candidate in the patient population. Before a clinical trial can be conducted in a European Member State, it must be approved by that Member State. The trial sponsor therefore submits a clinical trial application, containing clinical and non-clinical data on the product under investigation. It includes a protocol describing the objectives, design, and methodology of the trial, details on how the product will be manufactured (according to Good Manufacturing Practices (GMP)), and information on patient recruitment and informed consent. A scientific evaluation by the competent authority and an ethical review of the dossier by the Ethical Committee are carried out to assess whether the trial can be authorised.
In 2022, a new regulation for conducting clinical trials in the EU was implemented. It is called the Clinical Trials Regulation (CTR) and will replace the Clinical Trials Directive. The aim of this new regulation is to simplify and harmonise the procedures for assessment and supervision of clinical trials. It allows the organisation to submit an online application to up to 30 European Member States via one single online platform known as the Clinical Trials Information System (CTIS), in order to obtain authorisation in one or more Member States. There is currently a transition period for sponsors to implement the new regulation. Understandably, this poses quite a challenge for the parties involved.
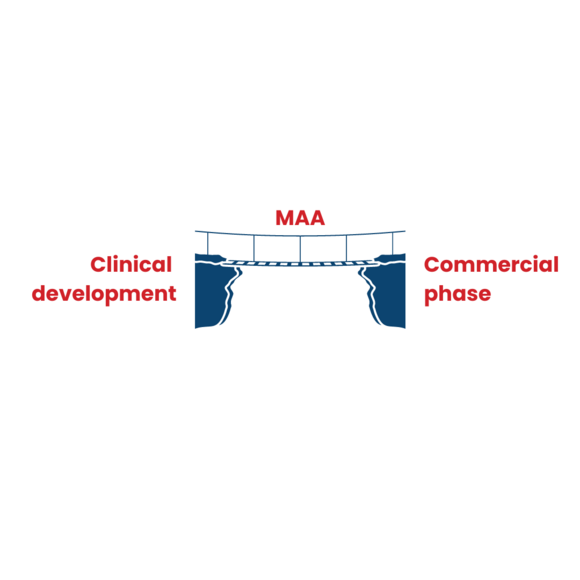
2. Marketing Authorisation: the bridge between clinical development phase and availability on the market
During clinical development, patient access to a drug is strictly controlled. A marketing authorisation is required before a drug can be distributed and sold on the market, prescribed by doctors and used by patients. The marketing authorisation therefore bridges the gap between clinical development and the commercial phase.
If the clinical studies demonstrated that the product is safe and effective, the sponsor can apply to the competent health authority for marketing authorisation. There are different regulatory procedures that differ in the applicability of the resulting marketing authorisation. For example, the centralised procedure results in a single marketing authorisation that is valid and binding in all Member States, while a national procedure is used to register the product in an single Member State.
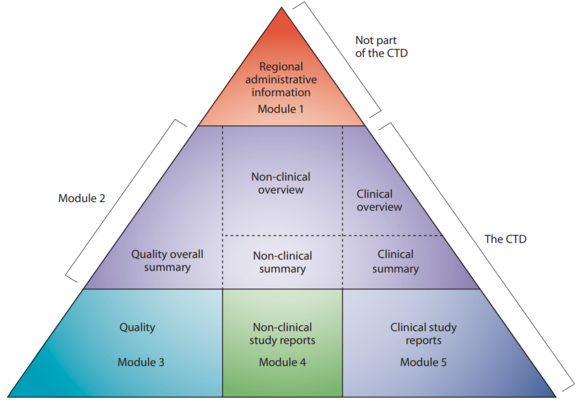
The Common Technical Document (CTD) is the core of the MAA. Although also (partially) applicable for clinical trial applications, it now contains all the data relating to the quality, safety and efficacy of the product to be marketed. The structure and format of the CTD has been established within the ICH framework.
Legend Structure of CTD
Module 1 Region specific and provides administrative data
Module 2 Summaries of Modules 3, 4 and 5
Module 3 Quality aspects
Module 4 Full reports of the nonclinical studies
Module 5 Full reports of the clinical
3. Managing variations to the registration dossier
During its life cycle, updates to the marketing authorisation may be needed. This includes, for example, changes to the product manufacturing process (e.g. equipment, test methods, components,…). As part of the GMP change control process, it is necessary to assess whether the planned change directly affects the information in the approved registration dossier. If so, an update of the registration dossier must be submitted to the competent authority of the Member State concerned. Such a change to the terms of the marketing authorisation is called a variation.
There are different types of variations:
· Type IA variation: A Type IA variation (“Do and Tell”) has minimal, if any, impact on the quality, safety or efficacy of the medicinal product. Notification to the health authority can be made after implementation, as no scientific evaluation is required.
An example of Type IA variation is a tightening of the specification limits of the finished product.
· Type IB variation: A Type IB variation (“Tell, Wait and Do”) must first be notified to the health authority. There is a 30-day waiting period before implementation for evaluation by the health authority, but no formal approval is required.
An example of a Type IB variation is the addition of a new manufacturer.
· Type II variation: A Type II variation is a major change that may have a significant impact on the quality, safety or efficacy of the medicinal product and may require a strategic planning for the implementation. The supporting documentation must be more extensive and the evaluation by the health authority will take longer (90 days).
An example of a Type II variation is the addition of a new therapeutic indication.
· Extension: An extension is a change that significantly alters the terms of the marketing authorisation and cannot be handled through the variation procedure. Consequently, a separate extension application must be submitted to the health authority, which may lead to an extension of the existing marketing authorisation or to a new marketing authorisation.
An example of an extension is a change or addition of a new route of administration or a new strength/potency.
Conclusion
In conclusion, we have highlighted the importance of regulatory processes at different phases of the development of medicinal products, from the clinical phase to the post-commercial phase which includes the variations. Regulatory affairs is an essential part of the drug lifecycle and ensures that only safe, effective and high quality drugs are marketed and reach the general public.

