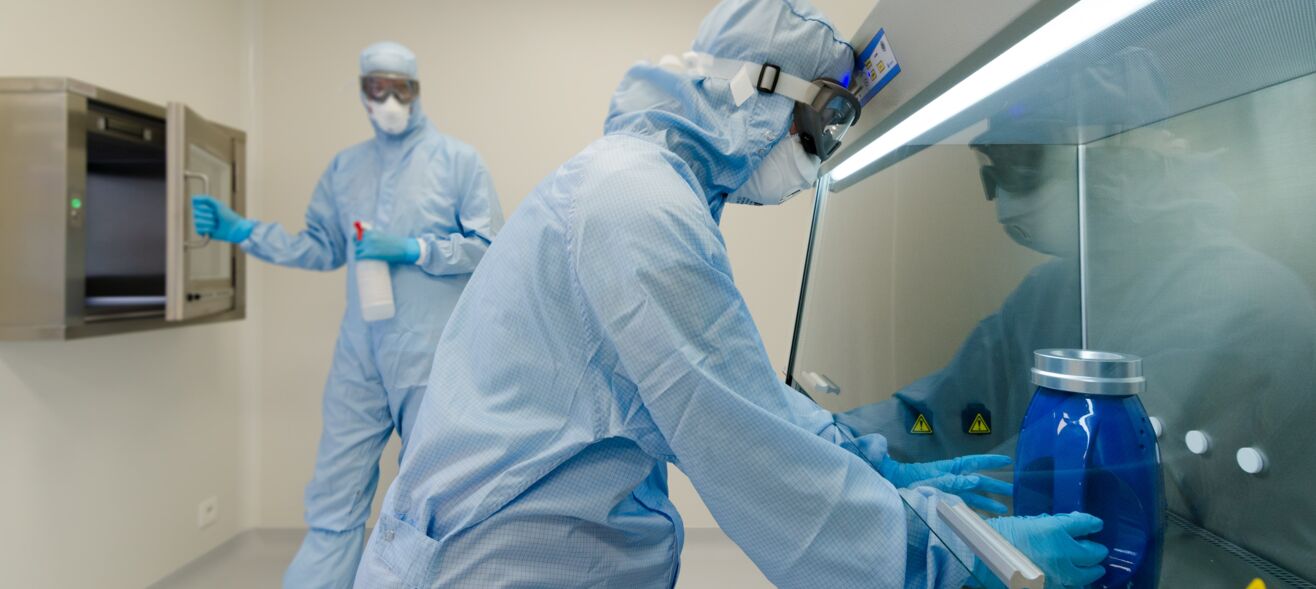
Learn about our approach
The pharmaceutical sector plays an essential role in advancing global health through continuous innovation and scientific research. At Advipro, we are proud to contribute by consistently providing the right expertise for every organization. Our experts offer a unique perspective on the industry and its challenges, guiding you in optimizing pharmaceutical processes. But how exactly do we do that?
Product development
Our experts in Quality Control, Quality Assurance, and Product Support are trained to optimize pharmaceutical product development. They delve into existing manufacturing technologies with you, offering advanced insights on how to elevate your position as a key player in the pharma industry. Importantly, Advipro's experts are well-versed in current quality standards and how to maintain them.

Quality control
Delivering high-quality pharmaceutical products? We achieve this by continuously monitoring all utilities and installations, and subjecting delivered products to rigorous quality controls. Through a combination of validation methods and monitoring, we consistently strive for optimal quality, meeting the standards expected in the pharma industry.
Regulations & compliance
The stringent standards and conditions for developing and manufacturing pharmaceutical products are crucial for ensuring the safety of patients and users. That's why we prioritize up-to-date knowledge and application of compliance. We analyze, test, and validate processes, facilities, and labs in the pharmaceutical industry to ensure full compliance with all pharmaceutical regulations."
Innovations and Future
At Advipro, we prioritize validation and quality control to ensure current processes run smoothly and safely. However, we also place significant value on optimizations and innovations for the future. The pharmaceutical industry evolves rapidly, and our experts consider it vital to stay updated with the latest developments. This enables us to assist companies in meeting and keeping pace with the dynamic sector.

Collaborating with stakeholders in pharmaceutical companies
Depending on the projects where our experts are involved, we collaborate with various contacts within large pharmaceutical organizations.
(Project) Managers or Intermediaries
Our consultants and experts are often called upon for highly specific projects. In these cases, direct communication typically occurs with the project manager or responsible individual who is most informed about the collaboration expectations.
HR Department or HR Manager
In consulting, where temporary expertise is brought in for specific projects, HR often plays a crucial role. At Advipro, we frequently engage with the HR manager to ensure proper screening and find the right match for each project.
CEO or General Manager
Final decisions for project-based hires often rest with the CEO or at the executive level. Discussions usually begin with project stakeholders to identify project needs and how an Advipro expert can meet them effectively."